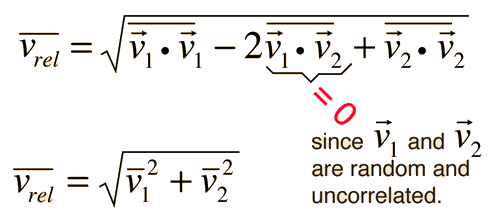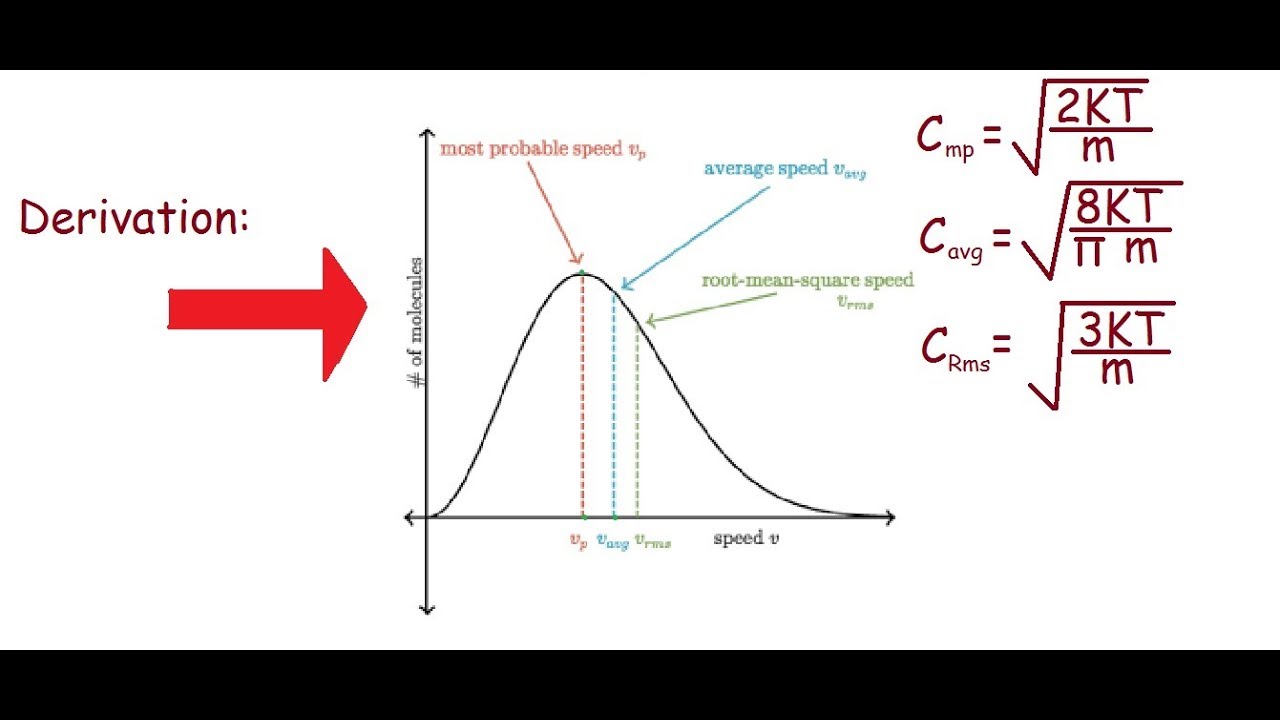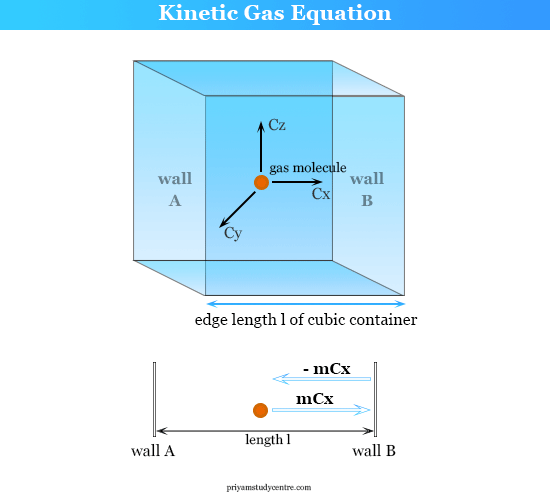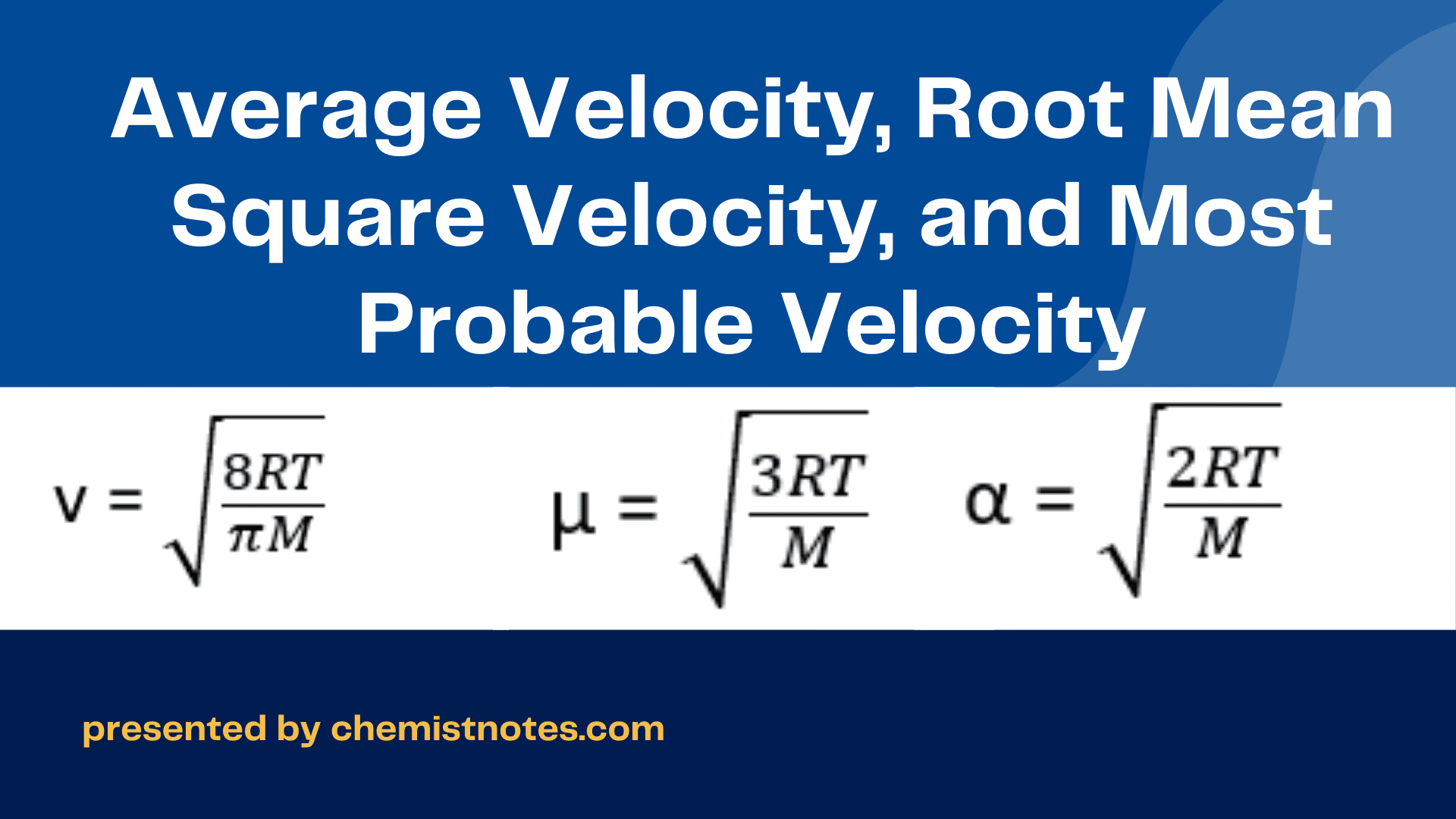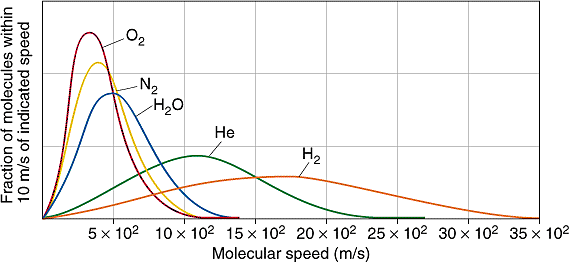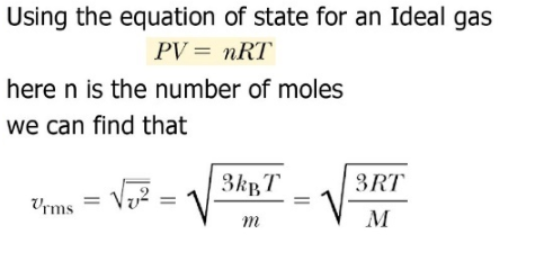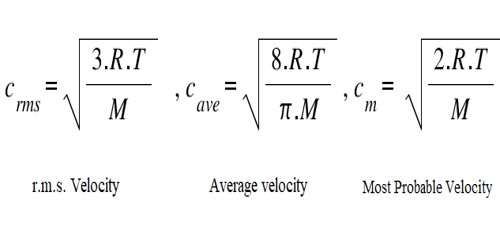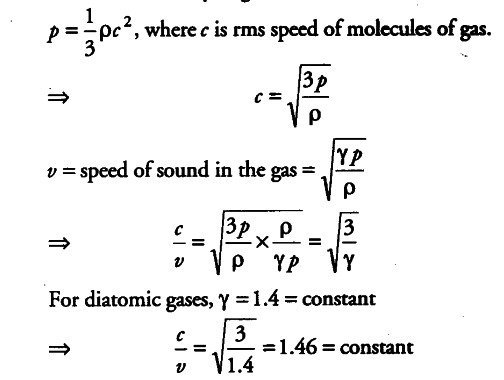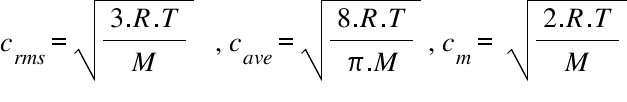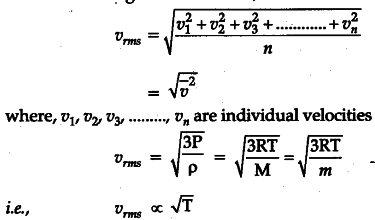
Define root mean square velocity of gas molecules give various relations for it - CBSE Class 11 Physics - Learn CBSE Forum

The average velocity of an ideal gas molecule at 27^oC is 0.3 m/s . The average velocity at 927^oC will be:
The average velocity of gas molecules is 400 m/s. Calculate its rms velocity at the same temperature. - Sarthaks eConnect | Largest Online Education Community
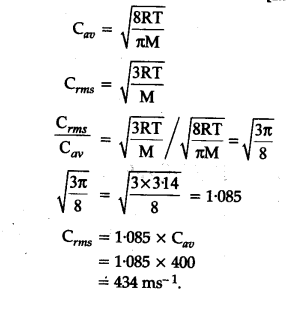
The average velocity of gas molecules is 400 m${{s}^{-1}}$.Calculate its r.m.s. velocity at the same temperature - CBSE Class 11 Chemistry - Learn CBSE Forum

What is average velocity of molecules of gas of molecular weight M at temperature T - Chemistry - Some Basic Concepts of Chemistry - 14208519 | Meritnation.com

The average velocity of an ideal gas molecule at 27^oC is 0.3 m/s . The average velocity at 927^oC will be:

At what temperature, the average speed of gas molecules be double of that at temperature, `27^(@)C`? - YouTube