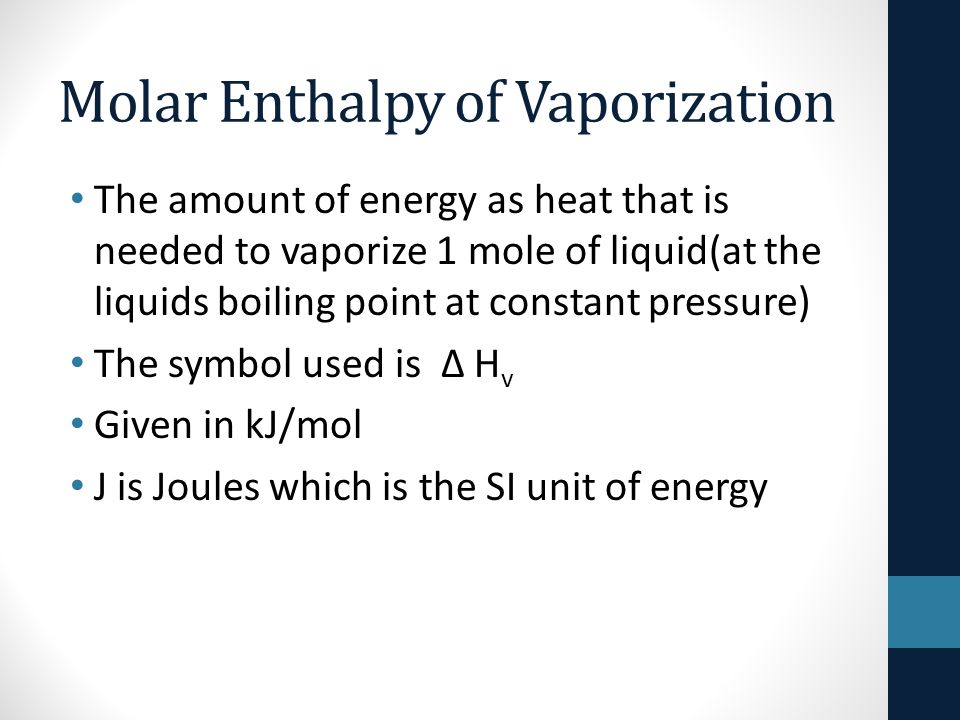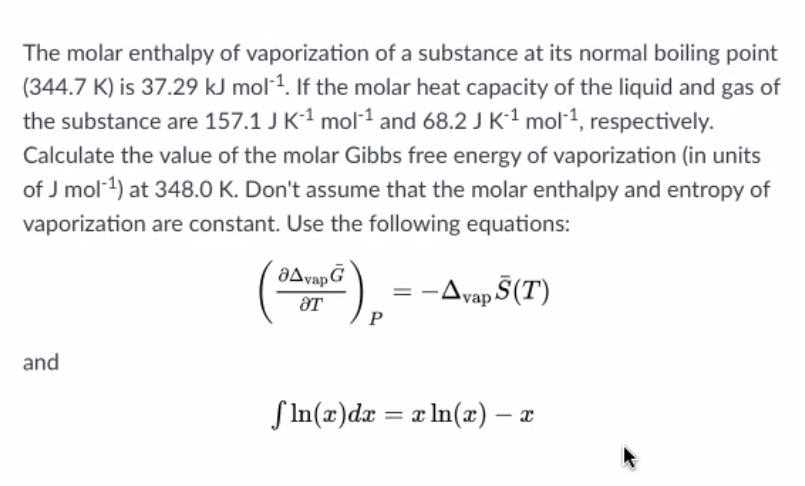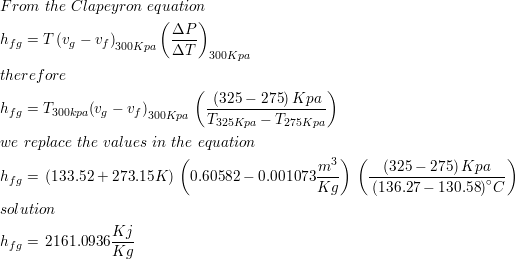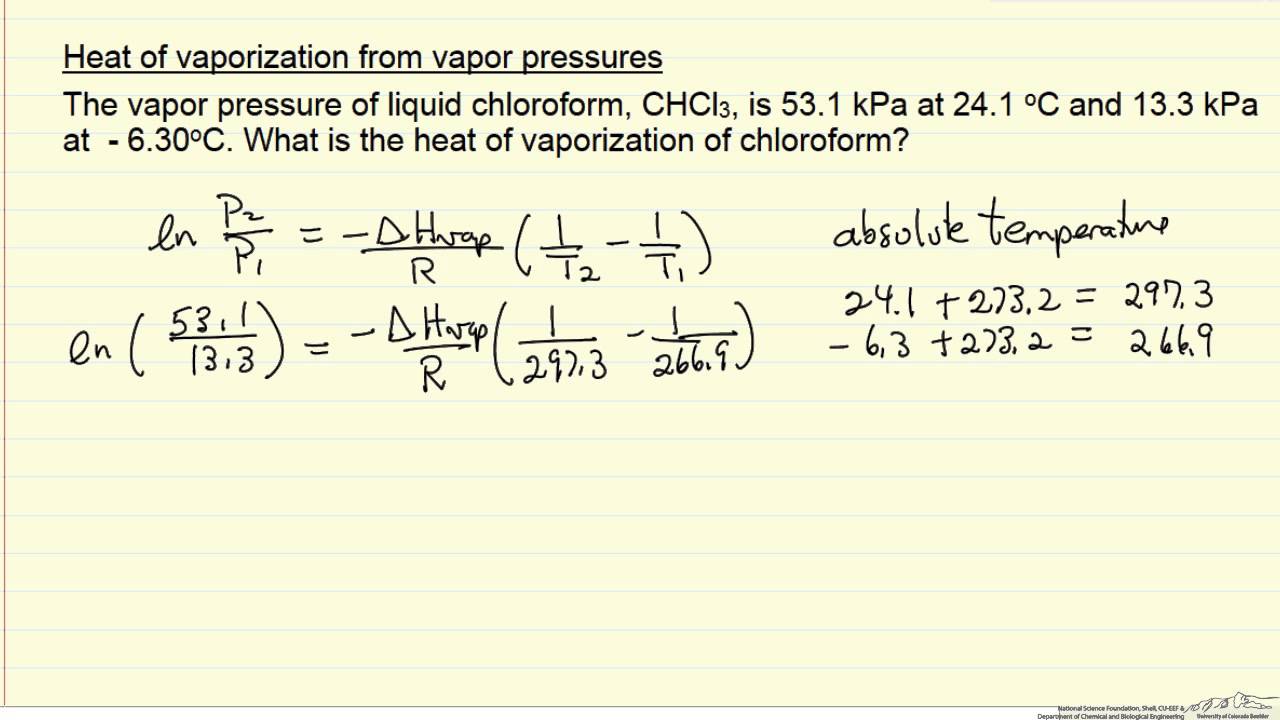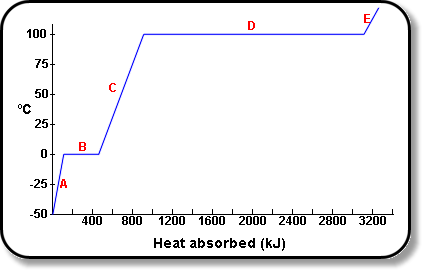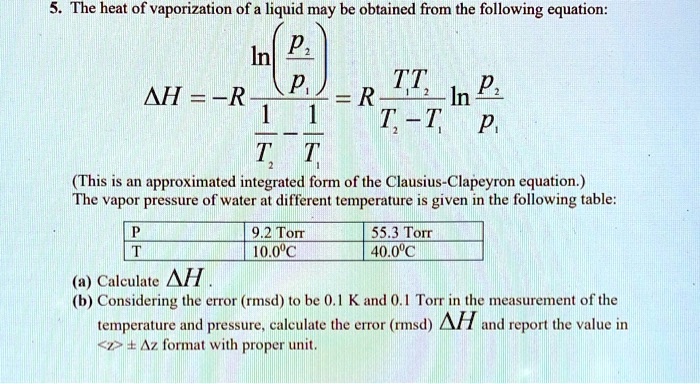
SOLVED: The heat of vaporization of. liquid may be obtained from the following equation: p; In p, TT AH = -R R In Pz T -T p (This is an approximated integrated
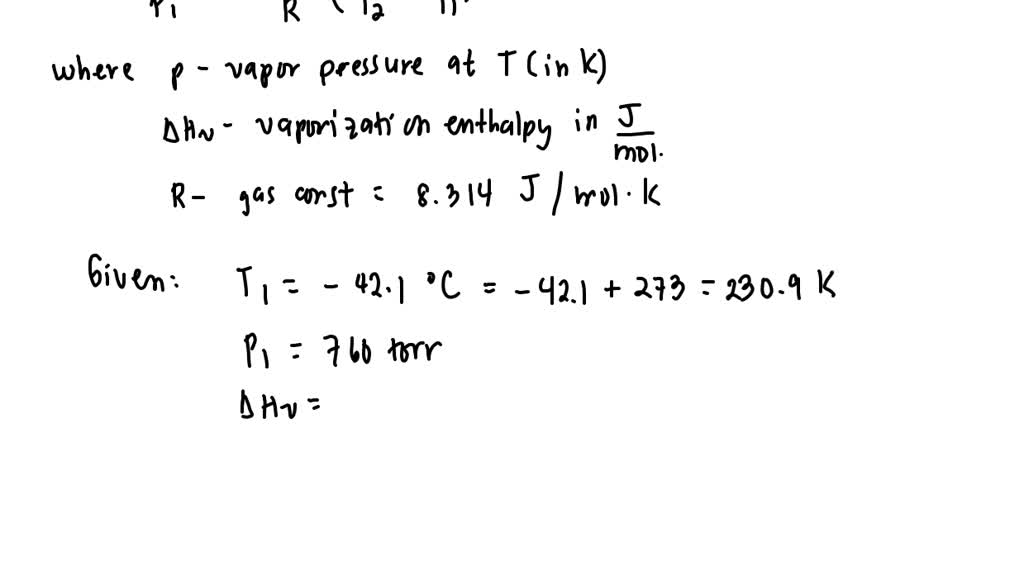
SOLVED: The enthalpy of vaporization of propane is 19.0 kJ/mol and its normal boiling point is -42.1 °C. Using the Clausius-Clapeyron equation, calculate the temperature at which propane has a vapor pressure
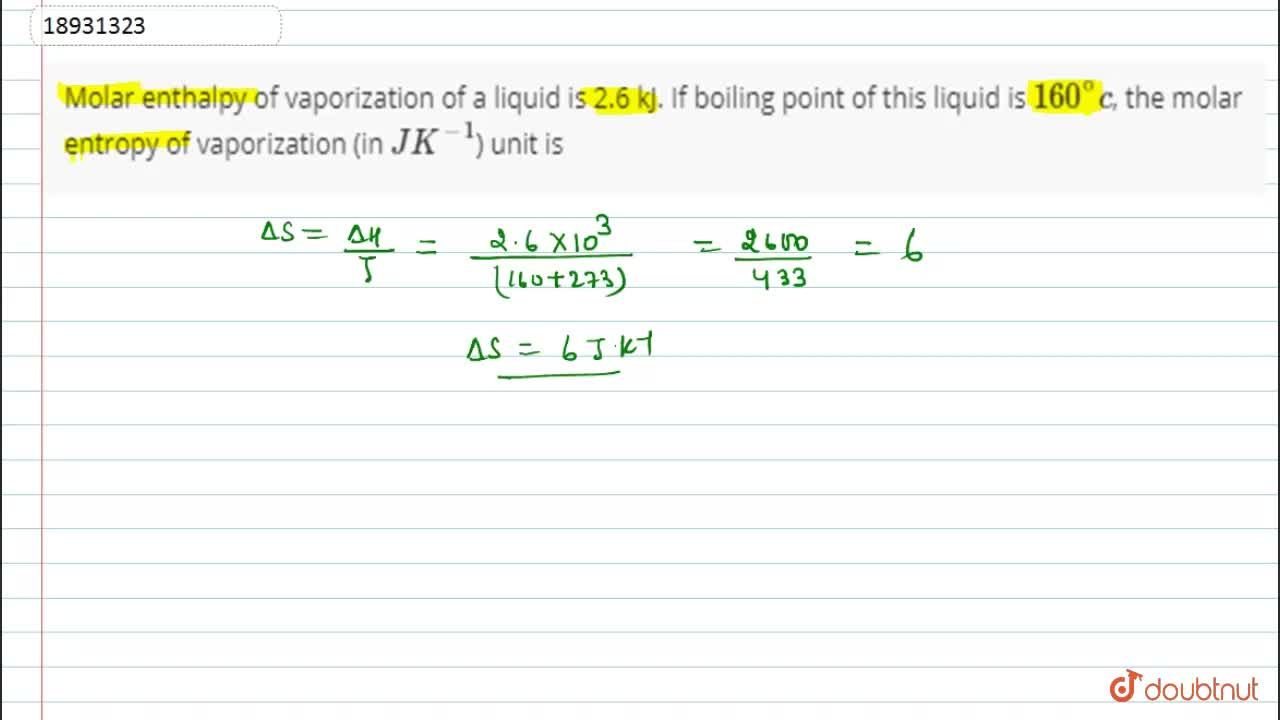
Molar enthalpy of vaporization of a liquid is 2.6 kJ. If boiling point of this liquid is 160^(@)c, the molar entropy of vaporization (in JK^(-1)) unit is
Calculate the molal elevation constant of water if molar enthalpy of vaporisation of water at 373 K is 40.585 kJ/mol.

Ethanol has a heat of vaporization of 38.56 kj/mol and a normal boiling point of 78.4 C - Home Work Help - Learn CBSE Forum

Ethanol has a heat of vaporization of 38.56 kj/mol and a normal boiling point of 78.4 C - Home Work Help - Learn CBSE Forum



-438.png)