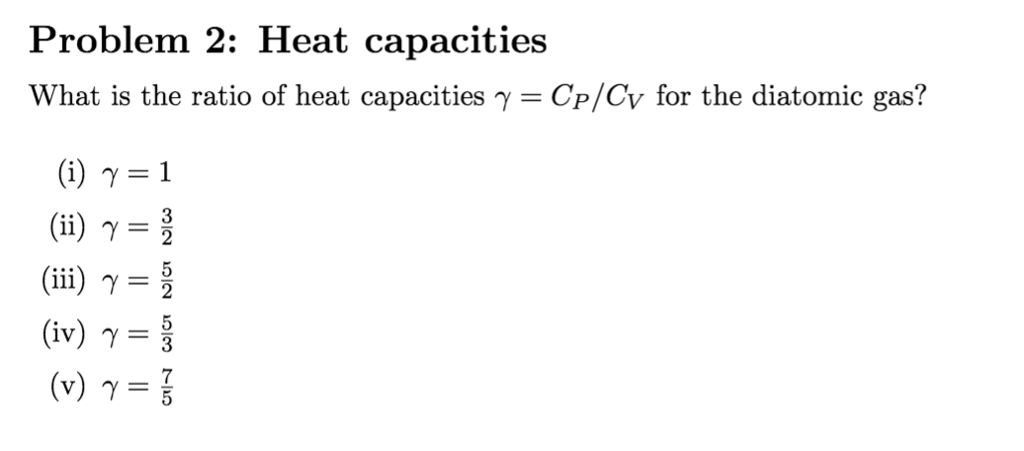If one mole of monoatomic gas is mixed with one mole of diatomic gas, what is the value of (Cp/Cv) for the mixture? - Quora

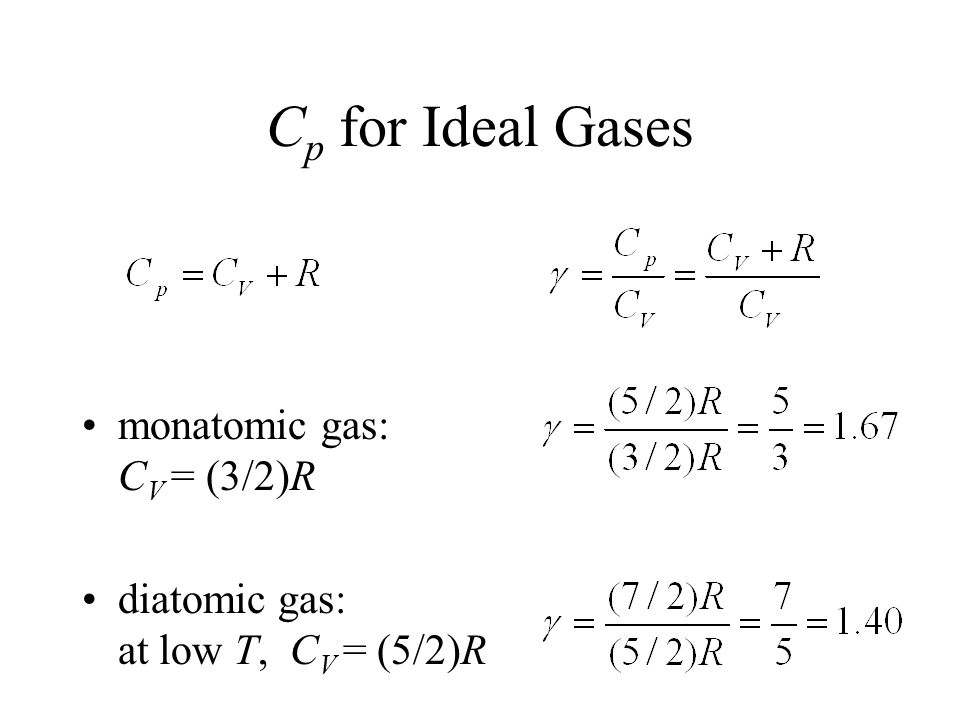
Cv and Cp denote the molar specific heat capacities of a gas at constant volume and constant pressure, respectively. Then
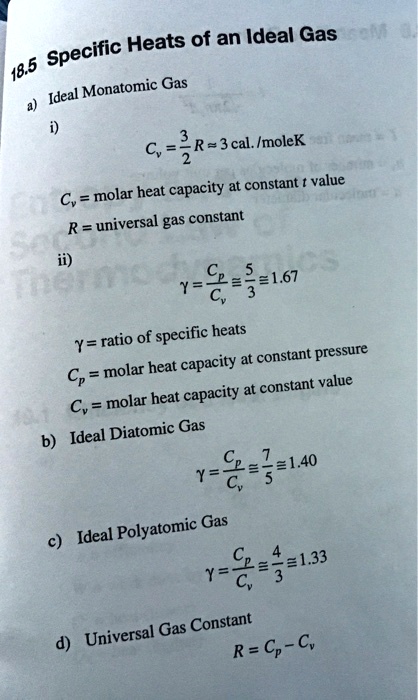
SOLVED: Heats of an Ideal Gas Specific 18.5 Monatomic ' Gas Ideal = C, 3 R=3cal.ImoleK at constant value molar heat capacity Cv= R= universal gas constant a 7-6,3e167 ratio specific heats
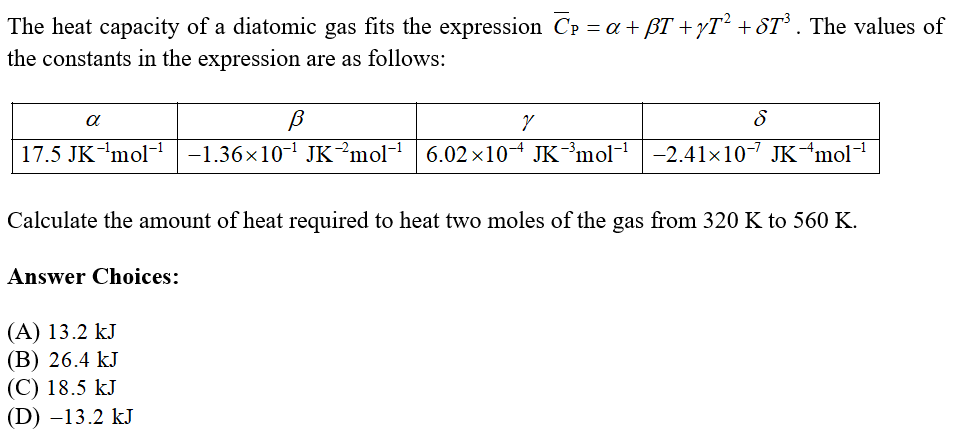
The value of( =Cp/Cv) , for hydrogen, helium and another ideal diatomic gas X (whose molecules are not rigid but have an additional vibrational mode), are respectively equal to :
Match the Cp/Cv ratio for ideal gases with different type of molecules Molecule Type Cp/Cv (A) Monatomic (I) 7/5 - Sarthaks eConnect | Largest Online Education Community
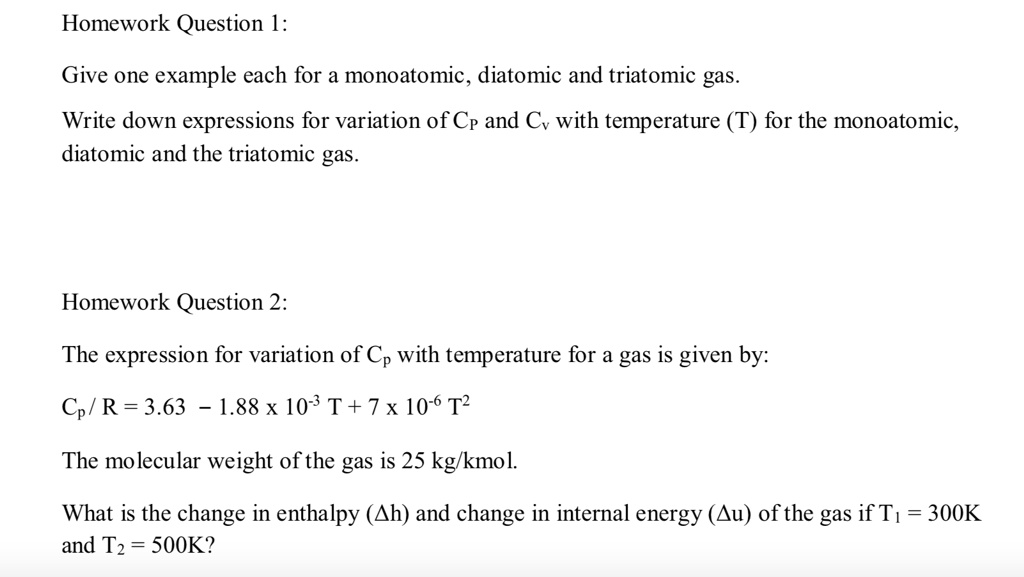
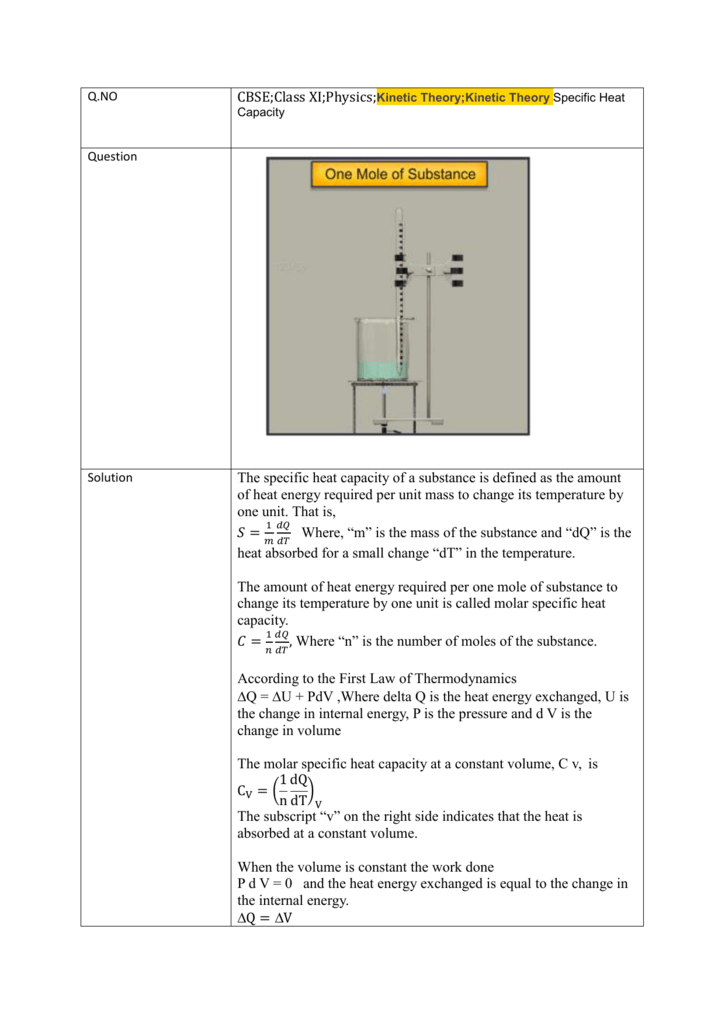
SOLVED: 1) Give one example each for a monoatomic, diatomic and triatomic gas. Write down expressions for variation of CP and Cv with temperature (T) for the monoatomic, diatomic and the triatomic

Cv and CP denote the molar specific heat capacities of a gas at constant volume and constant pressure respectively then - Physics - Kinetic Theory - 9969967 | Meritnation.com
If a monoatomic gas of 1 mol (γ = 5/3) is mixed with 1 mol of diatomic gas - Sarthaks eConnect | Largest Online Education Community
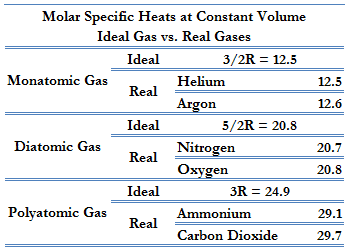
Derive the ratio of two specific heat capacities of monoatomic, diatomic and triatomic molecules - Sarthaks eConnect | Largest Online Education Community
126. When 1 mole of monatomic gas is mixed with 1 mole of diatomic gas then the equivalent value of Cp/Cv for the mixture will be (vibration mode neglected) A 1.33 B