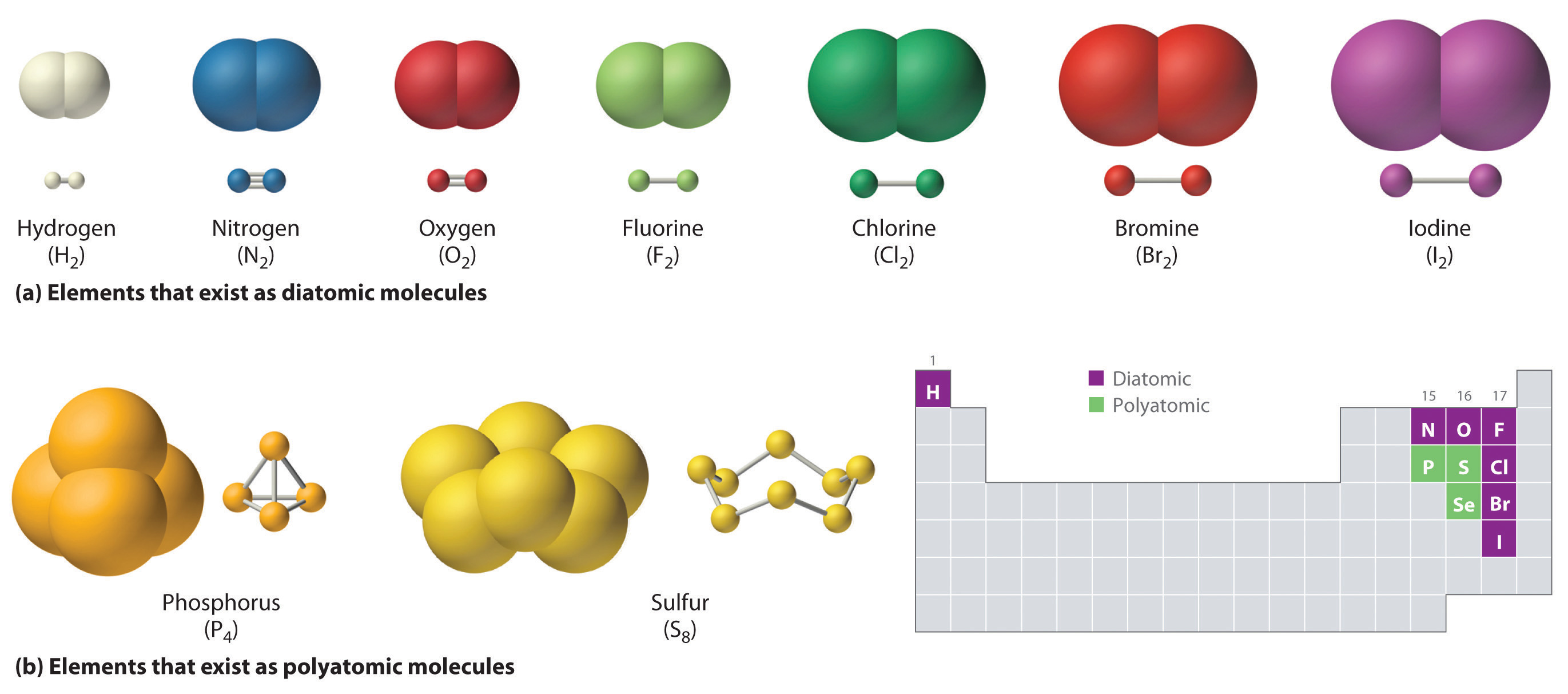
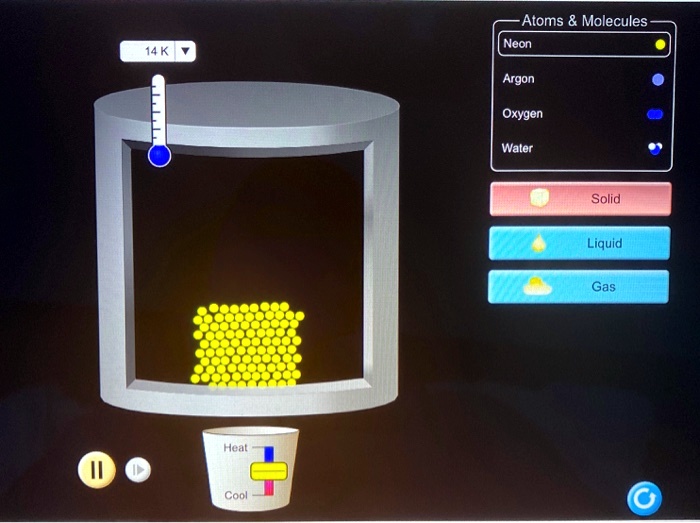
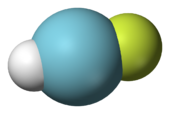Compilation of molecular diameters for small gases. (a) The van der... | Download Scientific Diagram

Argon Molecule. Scheme of Noble Gas Molecule Stock Illustration - Illustration of metal, neutron: 154012411
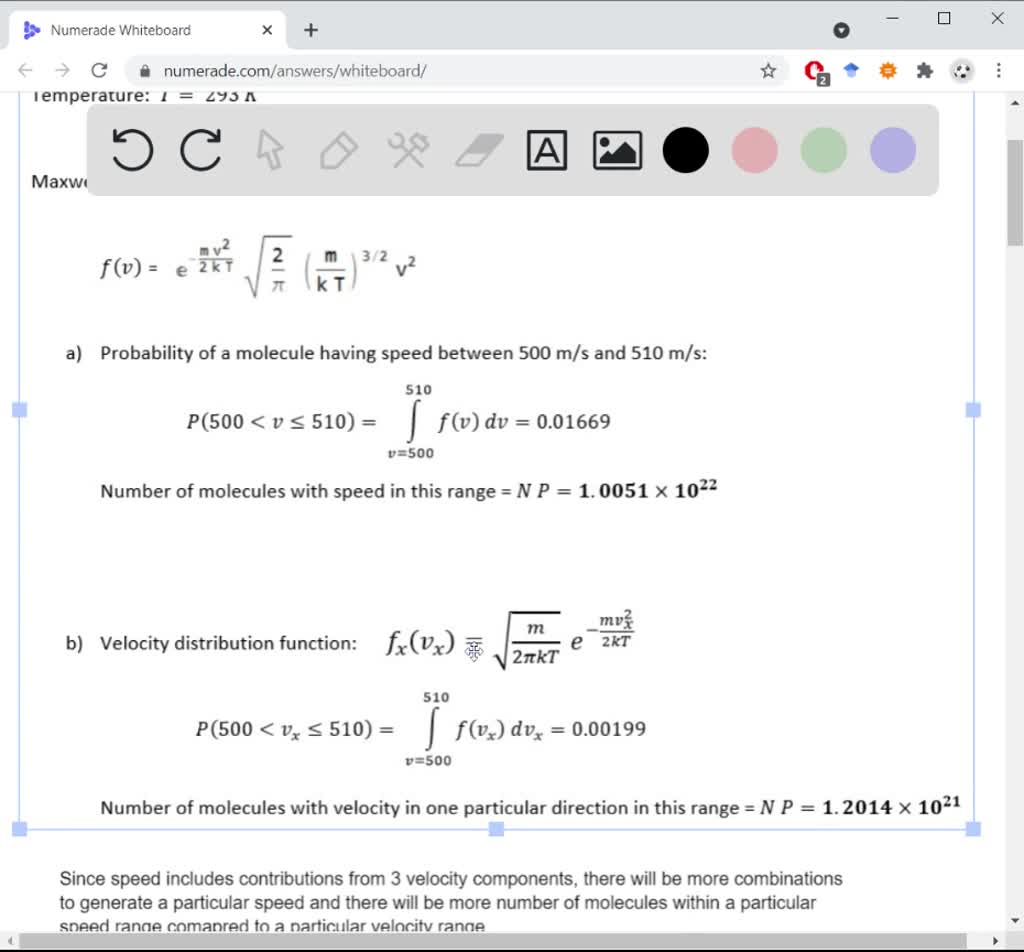
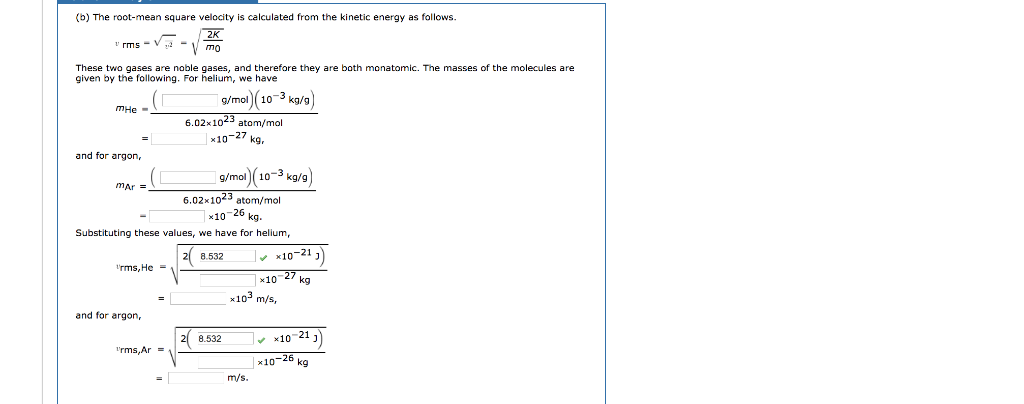
SOLVED:A cubic container holds one mole of argon gas at a temperature of 293 K. (a) How many molecules have speeds between 500 and 510 m / s ? ( b )

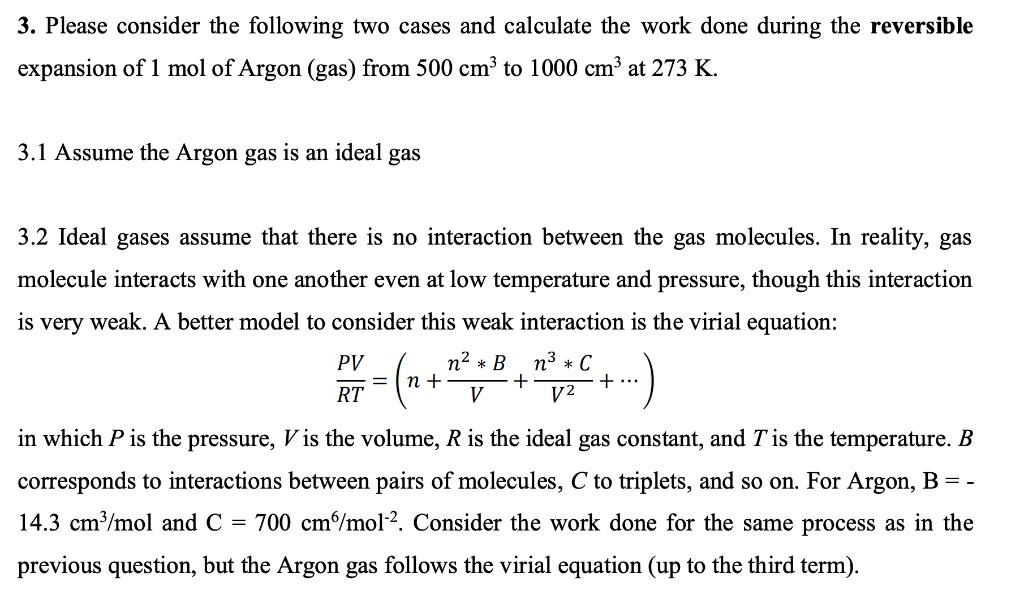
SOLVED: 1. Using the Maxwell-Boltzmann function, calculate the fraction of argon gas molecules with a speed of 305 m/s at 500 K. 2. If the system in problem 1 has 0.46 moles

Molecola Dell'argon Schema Della Molecola Del Gas Nobile Illustrazione di Stock - Illustrazione di sfondo, configurazione: 154336434

Molecular Models for the Hydrogen Age: Hydrogen, Nitrogen, Oxygen, Argon, and Water | Journal of Chemical & Engineering Data